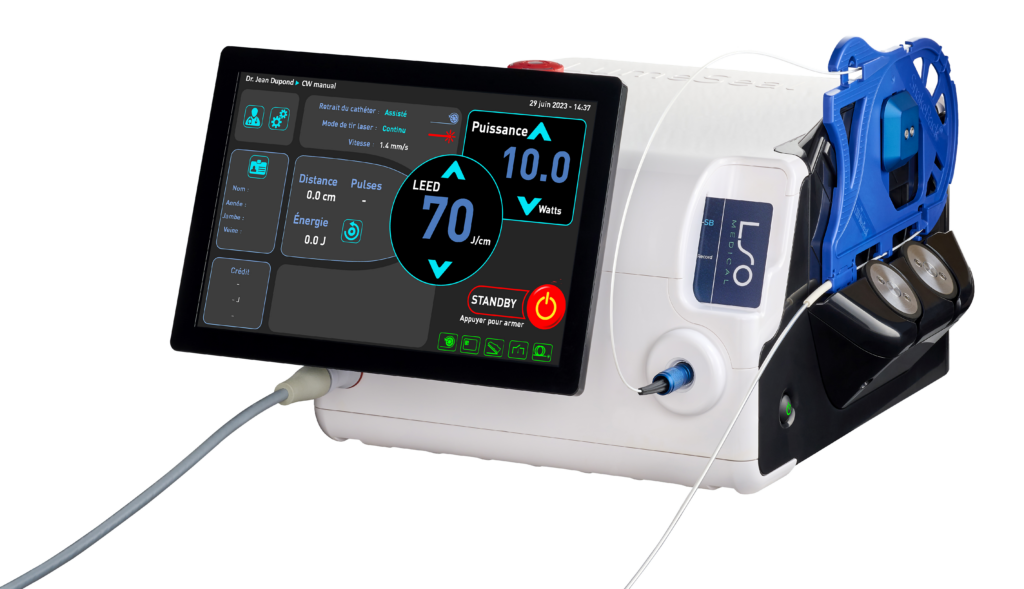
The 1470 nm endovenous laser for thermal ablation of varicose veins
- Occlusion of the great saphenous vein.
- Occlusion of the small saphenous vein.
Key features and compatible accessories
- Designed with SnakeBack® technology.
- Compatible with radial Ringlight_SB® fiber.
- Real-time auto-monitoring of the laser and fiber with the BackReflection™ system.
After the success of the Pharaoh 980 nm and following the validation of the clinical interest of the wavelength 1470 nm (Endotherme™ 1470), LSO Medical integrates a diode 1470 nm (infrared) in its new laser LumeSeal®.
This wavelength of 1470 nm combined with its “continuous” firing mode are now recognized as the Gold Standard in the thermal ablation treatment of saphenous by laser.
Since 2012, LSO Medical has been offering a complete range of Ringlight® radial fibers.
For added flexibility, Ringlight® radial fibers are available in 3 sizes:
- Standard 1.8 mm, 600 µm (+ option fused disponible)
- Slim Fused 1.3 mm, 400 μm
- Slim 1.0 mm, 400 μm
- Easy adjustment for better control of the procedure
- Assisted/automated fiber removal control managed by the user interface alone
The LumeSeal® incorporates the company’s latest patented innovation: SnakeBack® technology.
The SnakeBack® is a tension-free assisted removal system capable of planning, applying and controlling the delivery of energy into the vein.
The LumeSeal® thus allows a perfect delivery of energy, throughout the vein, maintaining a perfect regularity of treatment.
Complete traceability for each procedure:
- The LumeSeal® allows traceability of each procedure by the presence of a smart card delivered with each SB radial firing laser fiber.
- Laser: Laser Diode
- Wavelength: 1470 nm
- Laser class: 4
- Class DM: IIb (Directive 93/42/EEC and Regulation 2017/745/EU)
- Removal module: SnakeBack®
| Description | Reference | Technical specifications | Compatibility |
|---|---|---|---|
| RINGLIGHT® FIBER PROBE IR_SB 1,8 mm | ORLF000003_SB | Ringlight_SB® Fiber Standard 600 µm, 1.8 mm | Laser : LumeSeal® Introducer 6 Fr |
| RINGLIGHT® FIBER PROBE IRH_SB 1,0 mm | ORLF000005_SB | Ringlight_SB® Fiber Slim 400 µm, 1.0 mm | Laser : LumeSeal® Introducer 4 Fr or Cathlon 16G |
| RINGLIGHT® FIBER PROBE IR_SB 1,8 mm | ORLF000006_SB | Ringlight_SB® Fiber Fused Standard 600 µm, 1.8 mm | Laser : LumeSeal® Introducer 6 Fr |
| RINGLIGHT® FIBER PROBE IRH_SB 1,3 mm | ORLF000007_SB | Ringlight_SB® Fiber Fused Slim 400 µm, 1.3 mm | Laser : LumeSeal® Introducer 4 Fr or Cathlon 16G |
| Description | Reference | Technical specifications | Compatibility |
|---|---|---|---|
| Servante LumeSeal® | EV2-7-201 | Compatible LumeSeal_SB® | |
| Lunettes Praticiens | FG1#37 | Blue glasses lenses | |
| Lunettes Patients | IRD5#35 | Green glasses lenses |
| Description | Reference | Technical specifications | Compatibility |
|---|---|---|---|
| Dispenser DP 30 Pump – NOUVAG | 4187 |
| Nouvag 6022 / 6022a / 6022b tubing |
| Tubing – NOUVAG | 6022 / 6022a / 6022b | Length: 4 meters | Dispenser DP 30 Pump – NOUVAG |
| Vascular thermal ablation kit 6 Fr – LSO Medical | FR-K66189 | Ringlight® Fiber Standard | |
| Introduction kit 6 Fr – SCW | INTRO6F03818GS |
| Ringlight® Fiber Standard |
Destinations: vascular surgeons, vascular doctors, pharmacists, biomedical engineers and operating room staff.
Medical equipment Laser: Endotherme™ & LumeSeal_SB®. DM class: IIb. Laser class: 4
Disposables: Ringlight® and Ringlight_SB® Laser Fibers, DM Class: IIa.
![]() 1639
1639
Laser Consumables: Ringlight_SB® fibers are sold sterile. They are sterilized with ethylene oxide. They are designed for single use only. They must not be reused or resterilized.
Indications: Medical lasers and sterile disposables for endovascular coagulation, occlusion and removal of saphenous veins.
Benefits: The benefits of endovenous laser treatment are a size reduction or a complete obliteration of the treated varicose veins, and the improvement or even the resolution of venous symptoms. The risk of varicose veins complications will decrease significantly.
Contraindication: LumeSeal_SB® should not be used in the following cases: Patients with thrombi in the venous segment intended for treatment, Patients with an aneurysm in the venous segment intended for treatment, Patients with peripheral vascular diseases with an ankle-arm systolic pressure index below 0.9, Patients with deep vein thrombosis or with a history of deep vein thrombosis, Pregnant patients, Patients with infection on the area to be treated, active herpes or other viral infection, Other contraindications may possibly be indicated by the practitioner at the time of treatment. Read the instructions in the user manual carefully.
Adverse Effects: Based on the analyzed literature, three main categories of adverse effects can be identified for the laser procedure of GSV occlusion: Common adverse effects (incidence < 50%): bruising, pain, and superficial vein thrombosis. Rare adverse effects (incidence < 5% in most studies): deep vein thrombosis, paresthesia, and skin burns. Exceptional adverse effects (fewer than 10 cases reported): persistent paresthesia (lasting more than one year) and pulmonary embolism.
Device Use: Training in the use of LumeSeal® provided by LSO Medical or by individuals authorized by LSO Medical. Carefully read the instructions in the user manual. For the SnakeBack system to comply with the defined requirements, an annual replacement of the wheels and recalibration of the device must be performed, at least once a year. This maintenance must be carried out by a technician that has been officially certified by LSO MEDICAL or by its authorized local distributor. The non-compliance of this rule leads to the cancellation of the manufacturer guarantee.
Endovenous Laser Treatment: Covered by Health Insurance
Manufacturer: LSO Medical, Biocentre A. Fleming, Bât D – 280 rue Salvador Allende – 59120 Loos, France
Distributor and importer: SCW, NOUVAG
Internal reference: 23/04/LSOMEDICAL/PM/001
Version: IM EMB – VA-en

